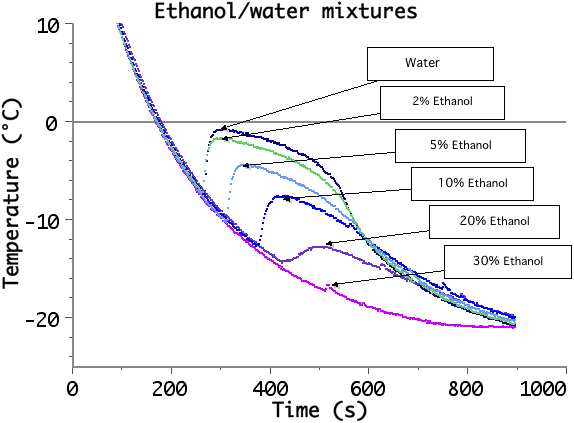
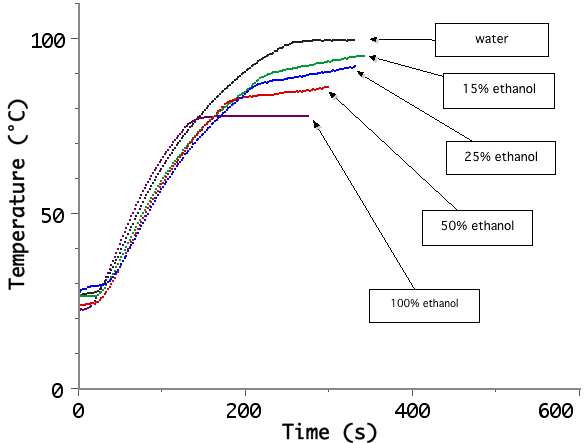
An educational kit, designed to study the behavior of a Peltier cell can also be used to investigate several interesting thermodynamics processes: liquid-solid and liquid-vapor phase transitions, supercooling, freezing point depression and boiling point elevation (using simple binary mixtures as salt/water, alcohol/water), latent heat of fusion measurements…
The kit consists of two coupled Peltier cells, connected in series, with the “hot” side kept at room temperature by a fan and with the “cold” side in contact with an aluminium cup (volume 10 cc) .
Both the hot and the cold sides have a temperature sensor, and a third temperature sensor measures the temperature of the liquid inside the cup.
The system is mounted on a plate with a series of connectors to carry out various measurements, including the study of the efficiency of the cells when used as power supply for a fan on a dc motor.
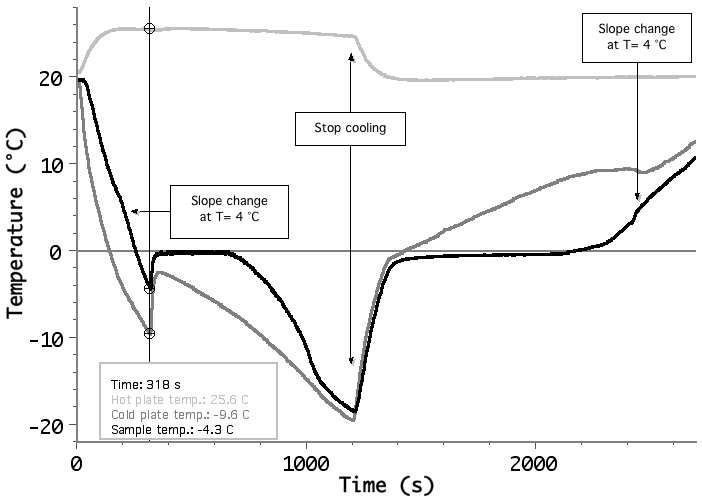
Example of supercooling of water: at the time t=318 s there is a discontinuity in the the water temperature (black line) showing liquid-solid phase transition which starts at T = -4.3 Celsius. Also the cup temperature heats-up suddenly (grey line). While ice is forming in the water volume, the temperature still slowly decreases (due to the finite temperature gradient) and when the transition is completed (around t=1000 s) the slope dT/dt increases, describing the cooling of solid water.
Using these data (knowing the water volume and the effective heat capacity of the system) it is possible to calculate the water latent heat of fusion.
By measuring current and voltage across a load (for example a motor driving a fan) when one side of the Peltier cell is heated, it is possible to estimate the efficiency of the process.